What Quantity of Energy Does It Take to Convert 1.29 Kg of Ice at
Learning Objectives
By the end of this section, you will be able to:
- Examine heat transfer.
- Calculate final temperature from heat transfer.
So far we accept discussed temperature change due to heat transfer. No temperature change occurs from heat transfer if ice melts and becomes liquid water (i.e., during a phase change). For example, consider h2o dripping from icicles melting on a roof warmed by the Lord's day. Conversely, water freezes in an ice tray cooled past lower-temperature surroundings.

Figure 1. Heat from the air transfers to the water ice causing it to melt. (credit: Mike Brand)
Free energy is required to melt a solid because the cohesive bonds between the molecules in the solid must exist broken apart such that, in the liquid, the molecules tin can move around at comparable kinetic energies; thus, there is no ascent in temperature. Similarly, energy is needed to vaporize a liquid, because molecules in a liquid collaborate with each other via bonny forces. There is no temperature change until a phase change is complete. The temperature of a cup of soda initially at 0ºC stays at 0ºC until all the water ice has melted. Conversely, energy is released during freezing and condensation, usually in the form of thermal free energy. Work is done by cohesive forces when molecules are brought together. The corresponding energy must be given off (dissipated) to allow them to stay together Effigy 2.
The energy involved in a phase alter depends on ii major factors: the number and strength of bonds or force pairs. The number of bonds is proportional to the number of molecules and thus to the mass of the sample. The strength of forces depends on the blazon of molecules. The estrus Q required to change the phase of a sample of mass 1000 is given by
Q =mL f(melting/freezing,
Q =mL v (vaporization/condensation),
where the latent estrus of fusion, L f, and latent rut of vaporization, Fifty v, are cloth constants that are adamant experimentally. See (Table 1).

Effigy 2. (a) Energy is required to partially overcome the bonny forces between molecules in a solid to form a liquid. That same energy must be removed for freezing to have place. (b) Molecules are separated by large distances when going from liquid to vapor, requiring significant energy to overcome molecular attraction. The same free energy must exist removed for condensation to take place. There is no temperature alter until a phase change is consummate.
Latent heat is measured in units of J/kg. Both L f and L v depend on the substance, particularly on the strength of its molecular forces equally noted earlier. Fifty f and L five are collectively called latent rut coefficients. They are latent, or subconscious, considering in phase changes, energy enters or leaves a system without causing a temperature modify in the system; so, in effect, the energy is hidden. Table ane lists representative values of 50 f and L v, together with melting and humid points.
The table shows that significant amounts of energy are involved in phase changes. Let us look, for example, at how much energy is needed to melt a kilogram of water ice at 0ºC to produce a kilogram of water at 0°C. Using the equation for a change in temperature and the value for water from Table 1, we find that Q =mL f = (1.0 kg)(334 kJ/kg) = 334 kJ is the energy to melt a kilogram of ice. This is a lot of free energy every bit it represents the aforementioned corporeality of free energy needed to raise the temperature of 1 kg of liquid water from 0ºC to 79.8ºC. Even more energy is required to vaporize water; it would have 2256 kJ to modify 1 kg of liquid water at the normal humid point (100ºC at atmospheric pressure) to steam (water vapor). This example shows that the energy for a phase change is enormous compared to energy associated with temperature changes without a stage change.
| Table one. Heats of Fusion and Vaporization[ane] | ||||||
|---|---|---|---|---|---|---|
| L f | Fifty v | |||||
| Substance | Melting point (ºC) | kJ/kg | kcal/kg | Boiling indicate (°C) | kJ/kg | kcal/kg |
| Helium | −269.7 | 5.23 | 1.25 | −268.9 | xx.ix | 4.99 |
| Hydrogen | −259.three | 58.6 | 14.0 | −252.9 | 452 | 108 |
| Nitrogen | −210.0 | 25.v | 6.09 | −195.viii | 201 | 48.0 |
| Oxygen | −218.8 | thirteen.viii | 3.thirty | −183.0 | 213 | fifty.9 |
| Ethanol | −114 | 104 | 24.9 | 78.3 | 854 | 204 |
| Ammonia | −75 | 108 | −33.4 | 1370 | 327 | |
| Mercury | −38.9 | xi.eight | two.82 | 357 | 272 | 65.0 |
| Water | 0.00 | 334 | 79.8 | 100.0 | 2256[2] | 539[iii] |
| Sulfur | 119 | 38.1 | 9.10 | 444.6 | 326 | 77.9 |
| Lead | 327 | 24.5 | five.85 | 1750 | 871 | 208 |
| Antimony | 631 | 165 | 39.four | 1440 | 561 | 134 |
| Aluminum | 660 | 380 | 90 | 2450 | 11400 | 2720 |
| Silver | 961 | 88.iii | 21.one | 2193 | 2336 | 558 |
| Gold | 1063 | 64.5 | 15.4 | 2660 | 1578 | 377 |
| Copper | 1083 | 134 | 32.0 | 2595 | 5069 | 1211 |
| Uranium | 1133 | 84 | xx | 3900 | 1900 | 454 |
| Tungsten | 3410 | 184 | 44 | 5900 | 4810 | 1150 |
Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and humid points, because evaporation and condensation (conversion of a gas into a liquid land) occur fifty-fifty at temperatures below the boiling point. Take, for case, the fact that air temperatures in humid climates rarely become above 35.0ºC, which is because most heat transfer goes into evaporating water into the air. Similarly, temperatures in boiling weather rarely fall below the dew betoken considering enormous oestrus is released when water vapor condenses.
Nosotros examine the effects of phase change more precisely by considering calculation heat into a sample of water ice at −20ºC (Figure 3). The temperature of the water ice rises linearly, arresting estrus at a constant rate of 0.l cal/g⋅ºC until it reaches 0ºC. Once at this temperature, the ice begins to cook until all the ice has melted, absorbing 79.8 cal/g of estrus. The temperature remains abiding at 0ºC during this phase modify. In one case all the ice has melted, the temperature of the liquid h2o rises, arresting heat at a new constant rate of i.00 cal/1000⋅ºC. At 100ºC, the water begins to boil and the temperature once again remains abiding while the water absorbs 539 cal/g of heat during this phase change. When all the liquid has become steam vapor, the temperature rises over again, absorbing rut at a rate of 0.482 cal/yard⋅ºC.
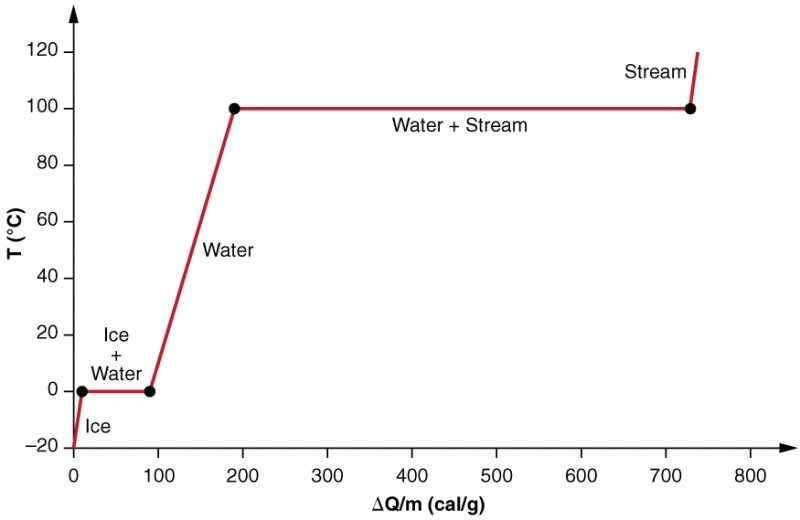
Figure 3. A graph of temperature versus energy added. The system is constructed and then that no vapor evaporates while ice warms to become liquid water, and so that, when vaporization occurs, the vapor remains in of the system. The long stretches of abiding temperature values at 0ºC and 100ºC reverberate the big latent heat of melting and vaporization, respectively.
Water tin evaporate at temperatures below the boiling bespeak. More energy is required than at the humid bespeak, because the kinetic energy of water molecules at temperatures below 100ºC is less than that at 100ºC, hence less free energy is bachelor from random thermal motions. Have, for example, the fact that, at body temperature, perspiration from the skin requires a oestrus input of 2428 kJ/kg, which is about x percentage college than the latent heat of vaporization at 100ºC. This heat comes from the skin, and thus provides an effective cooling mechanism in hot weather condition. High humidity inhibits evaporation, so that trunk temperature might rise, leaving unevaporated sweat on your forehead.
Instance 1. Calculate Final Temperature from Stage Change: Cooling Soda with Ice Cubes
Three ice cubes are used to chill a soda at 20ºC with mass chiliad soda = 0.25 kg. The ice is at 0ºC and each ice cube has a mass of 6.0 thousand. Assume that the soda is kept in a foam container so that heat loss can exist ignored. Assume the soda has the same rut capacity as water. Discover the final temperature when all ice has melted.
Strategy
The ice cubes are at the melting temperature of 0ºC. Heat is transferred from the soda to the ice for melting. Melting of water ice occurs in two steps: beginning the phase modify occurs and solid (ice) transforms into liquid water at the melting temperature, then the temperature of this water rises. Melting yields water at 0ºC, so more heat is transferred from the soda to this h2o until the water plus soda arrangement reaches thermal equilibrium,Q ice = −Q soda.
The heat transferred to the water ice is
Q water ice = m water iceL f + m ice c Due west(T f−0ºC).
The rut given off by the soda is Q soda = m soda c Westward(T f−20ºC). Since no heat is lost, Q ice = −Q soda, and so that
m iceFifty f + one thousand ice c W(T f−0ºC) = –thousand soda c Due west(T f−20ºC).
Bring all terms involving T f on the left-paw-side and all other terms on the right-hand-side. Solve for the unknown quantity T f:
[latex]\displaystyle{T}_{\text{f}}=\frac{m_{\text{soda}}c_{\text{Due west}}\left(20^{\circ}\text{C}\correct)-m_{\text{ice}}L_{\text{f}}}{\left(m_{\text{soda}}+m_{\text{ice}}\right)c_{\text{W}}}\\[/latex]
Solution
- Identify the known quantities. The mass of ice is chiliad ice = 3 × 6.0 g = 0.018 kg and the mass of soda is thousand soda = 0.25 kg.
- Calculate the terms in the numerator:m soda c W(20ºC)=(0.25 kg)(4186 J/kg ⋅ ºC)(20ºC) = 20,930 J andyard ice L f = (0.018 kg)(334,000 J/kg) = 6012 J.
- Calculate the denominator: (k soda +m ice)c W = (0.25 kg + 0.018 kg)(4186 M/(kg⋅ºC) = 1122 J/ºC.
- Summate the final temperature: [latex]\displaystyle{T}_{\text{f}}=\frac{xx,930\text{ J}-6012\text{ J}}{1122\text{ J/}^{\circ}\text{C}}=13^{\circ}\text{C}\\[/latex]
Give-and-take
This example illustrates the enormous energies involved during a stage alter. The mass of ice is nigh 7 percentage the mass of water just leads to a noticeable change in the temperature of soda. Although we causeless that the ice was at the freezing temperature, this is wrong: the typical temperature is −6ºC. Notwithstanding, this correction gives a final temperature that is substantially identical to the outcome nosotros found. Can you lot explain why?

Figure 4. Condensation on a glass of iced tea. (credit: Jenny Downing)
Nosotros have seen that vaporization requires heat transfer to a liquid from the environs, and so that free energy is released by the surroundings. Condensation is the reverse procedure, increasing the temperature of the surroundings. This increase may seem surprising, since nosotros associate condensation with cold objects—the drinking glass in the figure, for example. However, energy must be removed from the condensing molecules to make a vapor condense. The energy is exactly the same as that required to brand the phase change in the other direction, from liquid to vapor, and so it can be calculated from Q =mL v.
Condensation forms in Effigy 4 considering the temperature of the nearby air is reduced to beneath the dew bespeak. The air cannot hold as much water as it did at room temperature, and so water condenses. Energy is released when the water condenses, speeding the melting of the water ice in the glass.
Real-World Application
Free energy is also released when a liquid freezes. This phenomenon is used by fruit growers in Florida to protect oranges when the temperature is close to the freezing signal (0ºC). Growers spray water on the plants in orchards so that the h2o freezes and estrus is released to the growing oranges on the trees. This prevents the temperature within the orange from dropping beneath freezing, which would damage the fruit.

Figure 14.11. The ice on these trees released large amounts of energy when information technology froze, helping to prevent the temperature of the trees from dropping below 0ºC. H2o is intentionally sprayed on orchards to help prevent hard frosts. (credit: Hermann Hammer)
Sublimation is the transition from solid to vapor phase. Y'all may have noticed that snow can disappear into thin air without a trace of liquid h2o, or the disappearance of ice cubes in a freezer. The reverse is also true: Frost can grade on very cold windows without going through the liquid stage. A pop effect is the making of "fume" from dry ice, which is solid carbon dioxide. Sublimation occurs because the equilibrium vapor pressure of solids is not goose egg. Certain air fresheners employ the sublimation of a solid to inject a perfume into the room. Moth balls are a slightly toxic example of a phenol (an organic compound) that sublimates, while some solids, such as osmium tetroxide, are and then toxic that they must exist kept in sealed containers to prevent human exposure to their sublimation-produced vapors.

Effigy 5. Straight transitions between solid and vapor are mutual, sometimes useful, and fifty-fifty cute. (a) Dry ice sublimates directly to carbon dioxide gas. The visible vapor is made of h2o droplets. (credit: Windell Oskay) (b) Frost forms patterns on a very cold window, an case of a solid formed directly from a vapor. (credit: Liz W)
All phase transitions involve heat. In the case of direct solid-vapor transitions, the free energy required is given by the equation Q =mL s, where L s is the heat of sublimation, which is the energy required to change 1.00 kg of a substance from the solid phase to the vapor stage. Fifty south is analogous to L f and 50 v, and its value depends on the substance. Sublimation requires energy input, and then that dry ice is an effective coolant, whereas the opposite process (i.eastward., frosting) releases free energy. The corporeality of energy required for sublimation is of the same order of magnitude as that for other stage transitions.
The material presented in this section and the preceding section allows the states to calculate whatever number of effects related to temperature and stage modify. In each instance, it is necessary to identify which temperature and phase changes are taking place and so to apply the advisable equation. Go on in mind that oestrus transfer and work can cause both temperature and phase changes.
Problem-Solving Strategies for the Effects of Heat Transfer
- Examine the situation to determine that there is a modify in the temperature or phase. Is there heat transfer into or out of the arrangement? When the presence or absenteeism of a stage change is not obvious, you lot may wish to first solve the problem as if there were no phase changes, and examine the temperature modify obtained. If it is sufficient to have you past a boiling or melting point, you should then go back and do the problem in steps—temperature change, phase change, subsequent temperature change, and and then on.
- Identify and list all objects that modify temperature and phase.
- Identify exactly what needs to be determined in the problem (identify the unknowns). A written list is useful.
- Make a listing of what is given or what can exist inferred from the problem as stated (place the knowns).
- Solve the appropriate equation for the quantity to be determined (the unknown). If there is a temperature change, the transferred oestrus depends on the specific oestrus (see Table one in Temperature Change and Heat Capacity) whereas, for a stage modify, the transferred heat depends on the latent heat. Run into Tabular array ane.
- Substitute the knowns along with their units into the appropriate equation and obtain numerical solutions complete with units. You lot will demand to practice this in steps if there is more than 1 phase to the procedure (such as a temperature alter followed by a phase change).
- Bank check the answer to see if it is reasonable: Does it make sense? As an example, be certain that the temperature change does non also cause a phase change that you have not taken into business relationship.
Check Your Understanding
Why does snow remain on mountain slopes fifty-fifty when daytime temperatures are higher than the freezing temperature?
Solution
Snow is formed from ice crystals and thus is the solid phase of water. Because enormous rut is necessary for phase changes, it takes a certain corporeality of fourth dimension for this oestrus to be accumulated from the air, fifty-fifty if the air is in a higher place 0ºC. The warmer the air is, the faster this heat exchange occurs and the faster the snow melts.
Section Summary
- Most substances tin exist either in solid, liquid, and gas forms, which are referred to as "phases."
- Stage changes occur at fixed temperatures for a given substance at a given pressure, and these temperatures are called humid and freezing (or melting) points.
- During phase changes, heat absorbed or released is given past:Q =mLwhere L is the latent estrus coefficient.
Conceptual Questions
- Estrus transfer can crusade temperature and phase changes. What else can cause these changes?
- How does the latent rut of fusion of water help slow the decrease of air temperatures, possibly preventing temperatures from falling significantly below ºC, in the vicinity of big bodies of water?
- What is the temperature of ice right afterwards information technology is formed by freezing water?
- If yous identify ºC ice into ºC h2o in an insulated container, what volition happen? Will some water ice melt, will more water freeze, or will neither have place?
- What issue does condensation on a glass of ice water accept on the rate at which the ice melts? Will the condensation speed upwards the melting process or tiresome it down?
- In very humid climates where in that location are numerous bodies of water, such equally in Florida, information technology is unusual for temperatures to ascension to a higher place about 35ºC (95ºF). In deserts, however, temperatures tin rise far above this. Explain how the evaporation of water helps limit high temperatures in boiling climates.
- In winters, it is oftentimes warmer in San Francisco than in nearby Sacramento, 150 km inland. In summers, it is almost always hotter in Sacramento. Explicate how the bodies of water surrounding San Francisco moderate its extreme temperatures.
- Putting a lid on a humid pot greatly reduces the oestrus transfer necessary to keep it boiling. Explain why.
- Freeze-dried foods have been dehydrated in a vacuum. During the process, the food freezes and must exist heated to facilitate dehydration. Explicate both how the vacuum speeds up dehydration and why the food freezes as a result.
- When nevertheless air cools by radiating at night, it is unusual for temperatures to fall below the dew betoken. Explain why.
- In a physics classroom demonstration, an instructor inflates a balloon by oral cavity and so cools it in liquid nitrogen. When cold, the shrunken balloon has a small amount of light blueish liquid in it, likewise as some snow-like crystals. Every bit information technology warms up, the liquid boils, and part of the crystals sublimate, with some crystals lingering for awhile and then producing a liquid. Place the blue liquid and the 2 solids in the cold airship. Justify your identifications using data from Tabular array 1.
Problems & Exercises
- How much estrus transfer (in kilocalories) is required to thaw a 0.450-kg package of frozen vegetables originally at 0ºC if their heat of fusion is the same every bit that of water?
- A purse containing 0ºC ice is much more effective in arresting energy than one containing the same corporeality of 0ºC h2o. (a) How much heat transfer is necessary to raise the temperature of 0.800 kg of water from 0ºC to 30.0ºC? (b) How much estrus transfer is required to first melt 0.800 kg of 0ºC ice and and then raise its temperature? (c) Explain how your answer supports the contention that the water ice is more effective.
- (a) How much oestrus transfer is required to raise the temperature of a 0.750-kg aluminum pot containing 2.50 kg of water from xxx.0ºC to the boiling betoken and then boil away 0.750 kg of h2o? (b) How long does this have if the rate of heat transfer is 500 Due west ane watt = i joule/second (i W = 1 J/s)?
- The germination of condensation on a drinking glass of water ice water causes the ice to melt faster than information technology would otherwise. If eight.00 chiliad of condensation forms on a glass containing both water and 200 g of ice, how many grams of the ice will melt as a event? Presume no other heat transfer occurs.
- On a trip, yous notice that a 3.l-kg bag of ice lasts an boilerplate of one day in your cooler. What is the average power in watts entering the ice if it starts at 0ºC and completely melts to 0ºC water in exactly one mean solar day i watt = 1 joule/second (ane W = 1 J/s)?
- On a certain dry sunny mean solar day, a pond pool'due south temperature would ascension by 1.50ºC if not for evaporation. What fraction of the water must evaporate to comport away precisely enough energy to continue the temperature abiding?
- (a) How much heat transfer is necessary to raise the temperature of a 0.200-kg piece of ice from −20.0ºC to 130ºC, including the energy needed for phase changes? (b) How much time is required for each stage, assuming a constant 20.0 kJ/s rate of heat transfer? (c) Make a graph of temperature versus time for this procedure.
- In 1986, a gargantuan iceberg bankrupt away from the Ross Ice Shelf in Antarctica. It was approximately a rectangle 160 km long, 40.0 km wide, and 250 m thick. (a) What is the mass of this iceberg, given that the density of ice is 917 kg/m3? (b) How much rut transfer (in joules) is needed to melt information technology? (c) How many years would it take sunlight alone to melt ice this thick, if the ice absorbs an average of 100 Westward/m2, 12.00 h per twenty-four hours?
- How many grams of java must evaporate from 350 k of java in a 100-k glass loving cup to absurd the coffee from 95.0ºC to 45.0ºC? You may assume the java has the aforementioned thermal properties as water and that the average oestrus of vaporization is 2340 kJ/kg (560 cal/one thousand). (You may neglect the change in mass of the coffee as it cools, which volition give yous an answer that is slightly larger than correct.)
- (a) It is difficult to extinguish a burn on a crude oil tanker, because each liter of rough oil releases two.80 × 107 J of energy when burned. To illustrate this difficulty, calculate the number of liters of water that must exist expended to absorb the energy released by burning 1.00 L of crude oil, if the water has its temperature raised from 20.0ºC to 100ºC, it boils, and the resulting steam is raised to 300ºC. (b) Discuss boosted complications acquired past the fact that rough oil has a smaller density than water.
- The energy released from condensation in thunderstorms can be very large. Calculate the energy released into the temper for a minor storm of radius 1 km, assuming that 1.0 cm of rain is precipitated uniformly over this area.
- To help prevent frost damage, 4.00 kg of 0ºC water is sprayed onto a fruit tree. (a) How much heat transfer occurs every bit the h2o freezes? (b) How much would the temperature of the 200-kg tree decrease if this amount of estrus transferred from the tree? Take the specific heat to be iii.35 kJ/kg · ºC, and assume that no stage change occurs.
- A 0.250-kg aluminum bowl belongings 0.800 kg of soup at 25.0ºC is placed in a freezer. What is the final temperature if 377 kJ of energy is transferred from the bowl and soup, assuming the soup'south thermal backdrop are the same as that of h2o?
- A 0.0500-kg water ice cube at −30.0ºC is placed in 0.400 kg of 35.0ºC water in a very well-insulated container. What is the final temperature?
- If you pour 0.0100 kg of 20.0ºC water onto a 1.20-kg cake of ice (which is initially at −fifteen.0ºC), what is the final temperature? Yous may assume that the water cools and then apace that effects of the surroundings are negligible.
- Indigenous people sometimes cook in watertight baskets by placing hot rocks into h2o to bring it to a eddy. What mass of 500ºC rock must be placed in four.00 kg of xv.0ºC water to bring its temperature to 100ºC, if 0.0250 kg of water escapes as vapor from the initial sizzle? You may neglect the effects of the surroundings and take the average specific heat of the rocks to be that of granite.
- What would exist the last temperature of the pan and h2o in Calculating the Terminal Temperature When Heat Is Transferred Between 2 Bodies: Pouring Cold Water in a Hot Pan if 0.260 kg of water was placed in the pan and 0.0100 kg of the water evaporated immediately, leaving the remainder to come to a common temperature with the pan?
- In some countries, liquid nitrogen is used on dairy trucks instead of mechanical refrigerators. A 3.00-hour delivery trip requires 200 50 of liquid nitrogen, which has a density of 808 kg/miii. (a) Calculate the estrus transfer necessary to evaporate this amount of liquid nitrogen and enhance its temperature to 3.00ºC. (Use c p and presume it is constant over the temperature range.) This value is the corporeality of cooling the liquid nitrogen supplies. (b) What is this heat transfer rate in kilowatt-hours? (c) Compare the amount of cooling obtained from melting an identical mass of 0ºC ice with that from evaporating the liquid nitrogen.
- Some gun fanciers brand their own bullets, which involves melting and casting the atomic number 82 slugs. How much estrus transfer is needed to heighten the temperature and cook 0.500 kg of lead, starting from 25.0ºC?
Glossary
heat of sublimation: the energy required to alter a substance from the solid stage to the vapor phase
latent heat coefficient: a physical constant equal to the amount of heat transferred for every 1 kg of a substance during the change in phase of the substance
sublimation: the transition from the solid stage to the vapor phase
Selected Solutions to Problems & Exercises
1. 35.9 kcal
3. (a) 591 kcal; (b) 4.94 × 10iii s
5. 13.5 W
7. (a) 148 kcal; (b) 0.418 due south, three.34 s, 4.19 s, 22.6 s, 0.456 due south
9. 33.0 thou
10. (a) nine.67 L; (b) Rough oil is less dense than h2o, and so it floats on summit of the h2o, thereby exposing it to the oxygen in the air, which it uses to burn. Too, if the water is under the oil, it is less efficient in arresting the rut generated past the oil.
12. (a) 319 kcal; (b) 2.00ºC
14. 20.6ºC
16. iv.38 kg
18. (a) one.57 × 104 kcal; (b) 18.iii kW ⋅ h; (c) i.29 × 104 kcal
Source: https://courses.lumenlearning.com/physics/chapter/14-3-phase-change-and-latent-heat/